An ode to some brilliant discoveries that shaped modern science
through Structural Biology
Structural biology to me as an undergraduate student seemed like an untouchable enigma. Something with so much mathematics and precision was something that seemed daunting to me; however, that changed when I, like every other college student in India, was tasked with identifying Nobel Laureates in Biology and prepare projects on them. One of the scientists I therefore came across was Venki Ramakrishnan who has shared the Nobel with Ada Yonath and Thomas. A. Steitz for solving the structure of ribosomes; a biological machine that is responsible for the synthesis of proteins i.e. a protein machine. This elucidation was momentous as now not only did we already knew how DNA looked like and replicated but we were also successful in cracking the code for protein synthesis. Learning about these scientists and their extraordinary discovery was truly eye-opening for me. What did this mean for modern science?
The elucidation of the structure of DNA revolutionized modern science and inspired a whole generation of scientists to steer their interest towards the somewhat erratic and undisciplined life sciences. Life was deciphered through four letter codes, A, T, G and C. Now that the code of life was known, it could be manipulated by cutting or adding bases to the DNA as we pleased. Modern biology has given us this ability albeit with its shortcomings- CRISPR. This is a bacterial immune defence system that has been harnessed to modify genomes of organisms by using a cutting enzyme called the Cas9 enzyme which, with the help of another nucleic acid called RNA, identifies DNA sequences to be cut or deleted1. The cell detects a break and starts the cell’s repair process which can allow the cut part to be removed entirely, thereby causing an edit in the genome. One of the limitations of CRISPR-Cas technology is its preciseness; sometimes the edit may not happen where it is desired, or edits can happen in more places. Also, structural information of various components involved in the process have provided clues to make more precise edits and thereby developing precision medicine.


Figure 1. X-ray diffraction pattern and The Photo 51 taken by Dr. Rosalind Franklin.
Source: UK Research and Innovation Blog
The journey from discovering the structure of DNA to successfully mastering the technique of manipulating it has taken us several years. This impossible feat would not have been possible without one of the most important photographs in modern science taken by Rosalind Franklin titled ‘Photo 51’, an image of the predominant B-form of the DNA. This B-form of DNA and experimental conclusion coincided with Watson and Crick’s conclusions about DNA taking up helical structure that ultimately led the duo to win the 1962 Nobel Prize in Physiology or Medicine. This photograph was taken by a technique called X-ray diffraction that has now been surpassed by Nuclear Magnetic Resonance (NMR) and the latest Cryo-EM. The influence of structural biology has affected major pillars in modern science: elucidation of DNA structure at the molecular level, the structure of the ribosome at the transcriptomic level and so on. This has enabled us to structurally visualize the central dogma and led to the development of more precise gene editors and treatment of uncurable diseases such as diabetes. While Rosalind Franklin’s contributions have rightly shaped modern biology; through this article I also want to shed light on a similar figure who deserves a spotlight; Dorothy Hodgkin.
Dorothy Hodgkin, often credited with advancing X-ray crystallography, brought a deserved spotlight into structural biology when she solved the structure of a notoriously difficult molecule; vitamin B122. The molecule posed a unique challenge: owing to its high molecular size and the presence of an interesting component; a cobalt atom that earned it another synonym- cobalamin. The history of the discovery of cobalamin structure was due to spike in the number of cases of pernicious anemia, now understood to be caused by a deficiency of Vitamin B12, that was treated with liver concentrate which contained trace amounts of these molecules that alleviated the symptoms. The chemical structure of cobalamin enabled us to understand the functions of it as a cofactor particularly in its adenosylcobalamin form where it is responsible for fatty acid metabolism, amino acid metabolism in the mitochondria. The molecule structure enabled it to be stored in the body for as long as two years assisted in understanding the pathology associated with deficiencies such as pernicious anemia.

Fig. 2. Structure of Vitamin B12
Similarly, structural biology remains an indispensable part of modern biology with it being routinely used in drug development. With accurate knowledge of a particular molecules’ structure one can predict the interactions a drug can have with other compounds or how you can make the drug more efficient and smaller so it can be made into a tablet or a syrup based on its chemical composition. This process is usually combined with bioinformatic tools to predict the chemical’s behavior in-silico i.e. via computer simulations, which cannot be possible without the accurate structure information.

Fig. 3. Schoenmaker et.al, mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability, International Journal of Pharmaceutics, 15 May 2021.
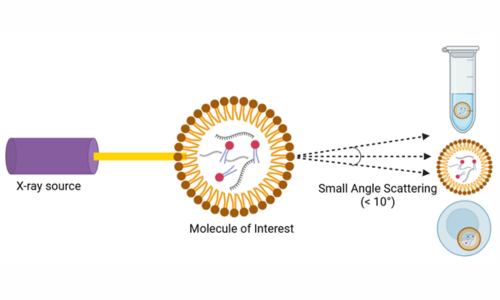
The development of the mRNA vaccine against the SARS-CoV2 used a technique called small-angle X-ray scattering (SAXS) to determine how the lipid droplets that enclose the mRNA take on optimal shapes, their behavior in solution and the release of the mRNA upon entry to human cells3. These techniques can also be utilized for understanding the protein architecture involved in the adsorption, entry and uncoating of the virus and release of the viral particles, hence aiding in the understanding of how the viruses diffuse across hosts.

Fig. 4. Small-angle X-ray scattering (SAXS)
While X-ray crystallography needed samples for the prediction of SARS-CoV2 virus, what if you did not need samples for seeing how the virus looked like but based on how similar viruses look, you could have the technology to predict it? With Alpha-Fold, that is where modern structural biology has now come down to. Alpha Fold, equipped with information of numerous proteins and their structures, can predict the structures of unknown proteins.
A dilemma in protein biology for decades was how a particular protein takes a particular shape. Afterall, proteins made of amino acids differing in numbers can take up various shapes, and this shape determination was extremely hard to predict and came to be known as the protein folding conundrum. However, the Alpha-Fold algorithm has been predicting highly complicated information of proteins with extreme accuracy4. Despite the accuracy, the algorithm suffers from the innovation that made the giant leaps in science possible, such as it being incapable of handling unspecified or novel domains and inaccurate prediction of metal domains such as zinc or cobalt coordination centers in a given predicted protein. Thus, a combination of AlphaFold2 based protein structure elucidation with traditional methods such as NMR spectroscopy and Cryo-EM would bring unprecedented development to the field while advancing us to the next big leap of modern biology: design and purification of proteins from scratch.
Structural Biology at its core sees proteins or membranes or cells at its singular level which has always been how biology came to be defined. Driven by curiosity and even more passion, Anton van Leewenhoek, saw animalcules made from lenses he crafted in his own workshop and thereby kickstarted a separate arena of science: Microbiology. Science has always been interdisciplinary; a massive tree of knowledge with various branches supporting each other. It remains to be seen how structural biology answers the many riddles and puzzles revolving around the question ‘How does it look like?’ to understand how a cell or a molecule works. Afterall, can you fully understand something, be it a cell or a protein, without seeing the entirety of it?
References:
- Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science (1979) 337, 816–821 (2012).
- Brink, C. et al. Structure of Vitamin B12: X-ray Crystallographic Evidence on the Structure of Vitamin B12. Nature 1954 174:4443 174, 1169–1171 (1954).
- Schoenmaker, L. et al. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int J Pharm 601, 120586 (2021).
- Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021 596:7873 596, 583–589 (2021).
. . .
Writer
Thara Bastion Antony
Editor
Bhargavi Nerikar
Illustrator
Shruti Morjaria