Just ranking above the population of anti-vaxxers around the world, water is the most abundantly found element on the Earth. Practically interwoven with every functional living being, we rely on water in countless ways.
(We would be more than happy to help you bust some Covid-19 vaccine-related myths if you fall in the anti-vaxxer category, or if you want to get to know more about it. The Science Paradox runs a regular column, The Viral Bulletin, where the readers will find reliable information about the pandemic and all strings attached to it.)
Water has been a consistent partygoer to all the major events that took place on Earth. One cannot stress more on its importance, and we surely owe the smooth operation of our body and existence to the holy H2O.
The emperor of all anomalies

The freezing point of water is 0°C, while its boiling point rests at 100°C.
There are certain instances in nature where one comes across various irregularities that produce astonishing results. Along with feisty teenagers, water also resorts to anomalous behaviour by acting up differently when the temperature goes from 4°C to 0°C. Instead of contracting, given the drop in the temperature, water expands between that range making it less dense. Well, teenagers may behave similarly if they fall back on the 420 trackways.
There’s more to add to this oddity. In a recent study conducted by a group of researchers at MIT, water is behaving more anomalously than ever. Michael Strano and his research group work with nanoparticles. Carbon nanotubes undeniably form the most minuscule spaces for any substance to gain entry in. These nanotubes, with an inner dimension not much bigger than an alleyway for a few water molecules to fit in, freeze water when it is trapped inside. Strano and his group found that water freezes solid inside these tubes even at high temperatures. Yes, temperatures that are more than the boiling point of water!
Water truly is a mysterious element, and this discovery has attracted a lot of scientists to the thought of possible applications on the peculiar behaviour of water. The fact that even familiar substances change their behaviour when subjected to compact structures that are measured in nanometer, has paved a path to manifesting innumerable possibilities.
Why is water going all jittery inside these carbon nanotubes?
Imagine a situation where someone puts you in the hole for 15 days straight. Most of us will reach the edge of losing our sanity.
Water is subjected to a similar situation when it is confined to nanometer-sized-pores. It exhibits unusual phase behaviour. This occurs due to the intermolecular pressure exerted by the container wall (carbon nanotube) on the confined water. The container wall can exert the equivalent of an additive or subtractive pressure on the fluid. This shifts the phase boundary of the fluid.
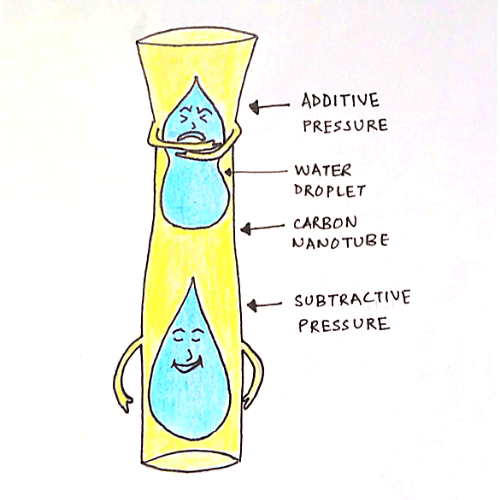
The most puzzling part of this story is the resistance of carbon nanotubes to water molecules. Carbon nanotubes are supposed to be hydrophobic. So, water molecules should not enter these tubes in the first place. Strano and his team are not able to find out the reason behind this surprise. Raman spectroscopy, Raman Radial Breathing mode (RBM) to be precise, and Vibrational spectroscopy were used to study the movement of confined water within the tube.
This peculiar behaviour of confined water is diameter specific, and huge ranges in the temperature shift are observed with changing diameters. Depending on the size of the tubes – shuttling between 1 to 2 nanometer – these water molecules line up in the right orientation, ready to make enough bonds with other water molecules. Interestingly enough, molecular simulations show that confined water freezes in carbon nanotubes even above 0°C. This property can be best used as a candidate for a latent heat thermal storage system.
Light at the end of the tunnel
Electric wires connect us with the rest of the world. Mapping almost every inhabited part of the Earth, electric wires have become an integral part of our lives. Although, we never think about its disastrous side when we switch on the lights every evening or call someone staying a few thousand miles away, electric wires have a huge impact on the environment.
The electricity system has become more complex than ever; spreading its roots right from complicated networks of power plants installed in every possible area to the widespread end-users of electricity who require electricity on the daily basis. These interconnected, transmission lines pose unseen threats to the environment. Visibly harmful, these power lines lie dangerously close to the trees. There have been multiple instances where irregular supply and abrupt stalling has caused these wires to catch fire, subsequently starting forest fires in densely vegetated areas. Power lines disturb untouched ecosystems when placed in underdeveloped areas. Around 40% of the global CO2 emission is due to the generation of electricity. Fossil fuels are combusted for their production, and these fuels produce CO2 when burnt. CO2 is the primary heat-trapping Greenhouse gas. Unfortunately, we are wound up in a disaster. Switching to a better, environment-friendly alternative is the need of the hour.
‘Ice Wires’ could be the key to solve this e-waste epidemic. Strano and his research group are looking at applications where they would be using ice-filled tubes as wires that will allow protons to move, similar to the movement of electrons in electric wires. Since these ice nanotubes sustain well and are stable at room temperature, it provides a more solid ground to actually start diving deep into the possibility of replacing electric wires with ‘ice wires’. These ‘ice wires’ would also prove to be a cost-effective replacement.
Water conducts protons 10 times more readily compared to any other conductive material. Here, the protons would not move along the length of the carbon nanotube. Instead, they will follow a mechanism quite similar to the domino effect. Say 10 protons are lined up in the wire. The first proton will push the second proton, resulting in a shuffle that will cascade down till the tenth proton which will eventually pop out.
Michael Strano and his research team might just start a new technological revolution if they can somehow make ‘Ice Wires’.
Why turn water into wine when you can pass it through carbon nanotubes and use it to transport electricity?
. . .
Writing and Illustration By

Urja Kuber
Co-founder and Director of Website development and Logistics
